
Regulatory Pathways to Market Bootcamp
Are you an early-stage start-up with an evolutionary idea for a digital health or medical device? Then the Regulatory Pathways to Market Bootcamp is for you.
This tailored Bootcamp provides you with specialised assistance to guide you through the highly regulated pathway of the European healthcare system whilst streamlining your innovation’s entry to the market.
Embark on this transformative 12-week online journey alongside industry experts, kicking off on 8 September and concluding on 28 November 2025. You’ll be assisted with insights crucial for identifying reimbursement routes, as well as expert guidance to craft a custom market strategy for your start-up to thrive.
Applications to Regulatory Pathways to Market Bootcamp are now closed. Sign up to our newsletter and be the first to know when a new challenge is available.
What you get
How it works
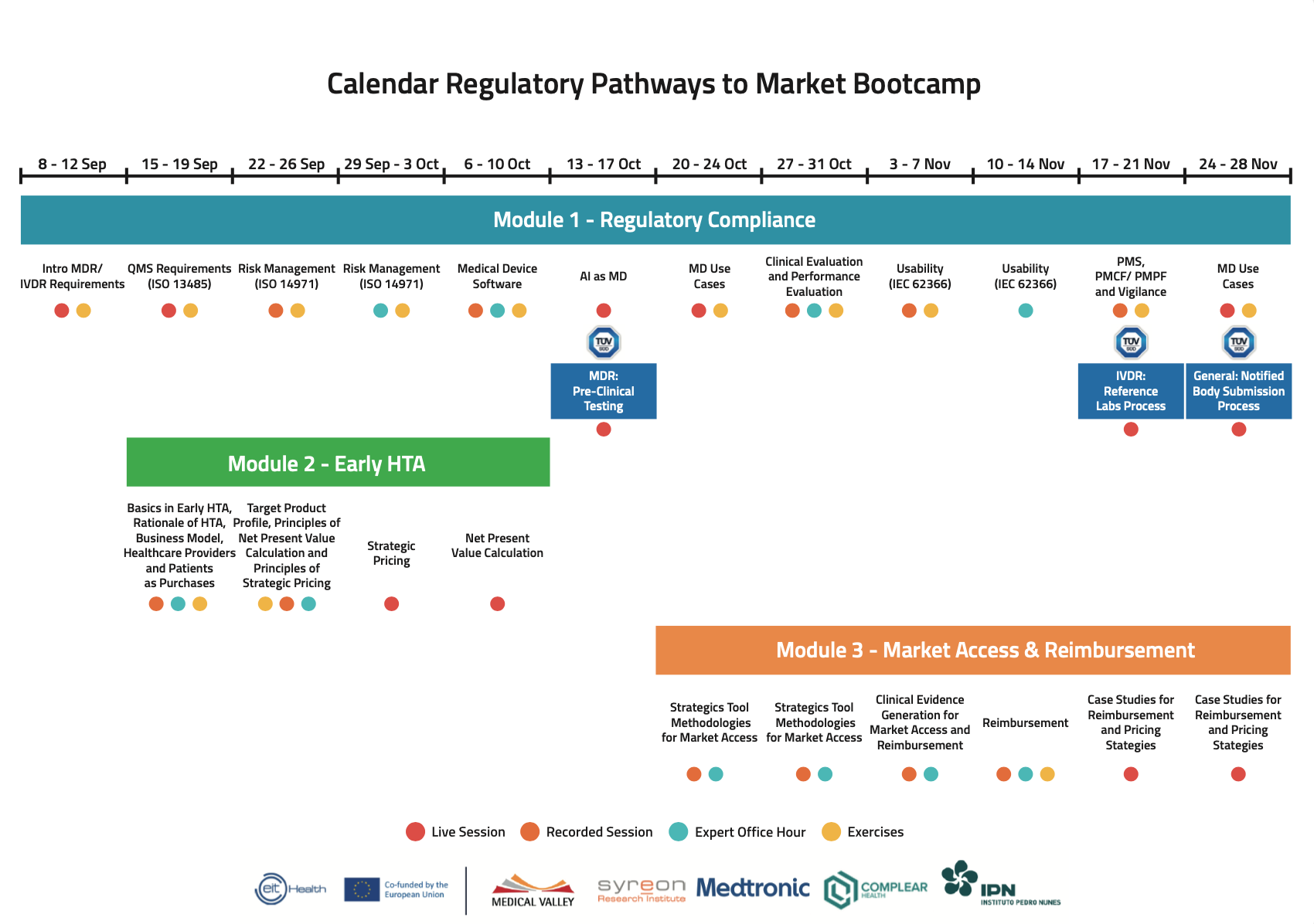
Dates of the process
Curriculum
Who should apply?
Ready to catapult your innovation into the heart of the European market?
We’d like to hear from you if you are a start-up or SME that:
- Is incorporated in a Horizon Europe country
- Are TRL 3-6
- Has experience with the technology and knowledge in HealthTech
Please note:
- Once accepted into the Bootcamp there is a commitment fee of €1.000,00. We will give a 50% discount to the top 10 companies classified according to their scores.
- The market value of this programme is €6.000,00. With EIT Health support, discounts are applied.
- Each company will have access to training for 2 participants.
- VAT will be added if applicable according to EU legislation.
Info Session
Webinars are a great way to find out if the programme is a right fit for you. Take a look at the webinar recording that took place in March 2025.
Watch recording.
Ready to apply?
Applications to Regulatory Pathways to Market Bootcamp are now closed. Sign up to our newsletter and be the first to know when a new challenge is available.
Sign up for updatesMeet the team
Partners
Sign up to our newsletter

By joining our mailing list you will:
- Be the first to know when this programme is open for applications
- Stay up to date about upcoming programmes and opportunities that may be a good fit for you










